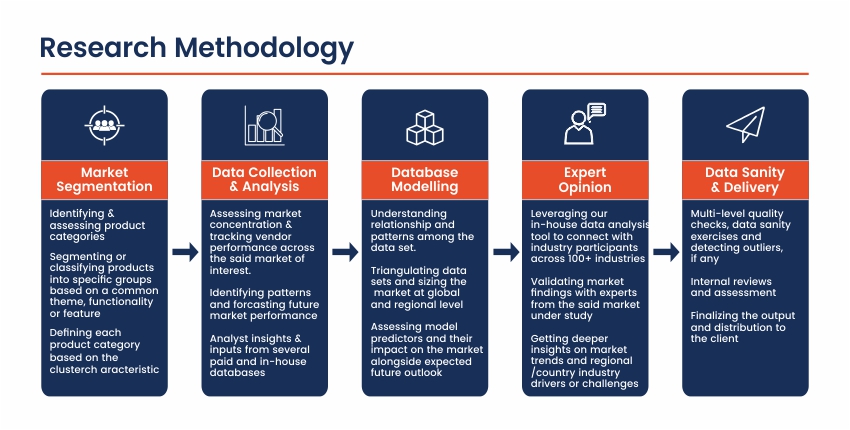
Market Overview
The South Korean medical device regulatory affairs market has witnessed significant growth in recent years, driven by the country’s strong emphasis on healthcare innovation, patient safety, and regulatory compliance. As a global leader in the medical device industry, South Korea has established a robust regulatory framework to ensure the quality, safety, and efficacy of medical devices sold within the domestic market, as well as those exported to international markets.
The medical device regulatory affairs market in South Korea encompasses a range of services and solutions that support medical device manufacturers, importers, and distributors in navigating the complex regulatory landscape. This includes regulatory strategy development, product registration and approval processes, post-market surveillance, and ongoing compliance management.
The South Korean medical device industry has been at the forefront of technological advancements, with a focus on innovative products and solutions that cater to the evolving healthcare needs of the country. This has led to a growing demand for specialized regulatory affairs expertise to help medical device companies navigate the intricacies of the regulatory system and bring their products to market in a timely and compliant manner.
The South Korean government, through various regulatory agencies and initiatives, has played a pivotal role in shaping the medical device regulatory affairs market. By continuously updating regulations, harmonizing standards, and promoting industry collaboration, the government has created an environment that fosters innovation and ensures the safety of medical devices for both domestic and international use.
Key Takeaways of the market
- Robust regulatory framework for medical devices in South Korea, ensuring quality, safety, and efficacy
- Growing demand for specialized regulatory affairs expertise to support medical device companies in navigating the complex regulatory landscape
- Emphasis on technological advancements and innovation in the South Korean medical device industry
- Ongoing government initiatives to update regulations, harmonize standards, and promote industry collaboration
- Increasing focus on post-market surveillance and compliance management to ensure the long-term safety and performance of medical devices
- Opportunities for regulatory affairs service providers to cater to the needs of both domestic and international medical device companies
- Challenges related to the integration of emerging technologies, such as digital health and AI-powered devices, into the existing regulatory framework
Market Drivers
The South Korean medical device regulatory affairs market is primarily driven by the country’s commitment to ensuring the safety and efficacy of medical devices available within the domestic market. As a global leader in the medical device industry, South Korea has established a robust regulatory framework that is continuously evolving to keep pace with technological advancements and changing healthcare needs.
One of the key drivers is the strong emphasis on innovation and the development of cutting-edge medical devices within the South Korean industry. As medical device companies strive to bring innovative products to market, the need for specialized regulatory affairs expertise has grown exponentially. These companies require support in navigating the complex approval processes, managing regulatory compliance, and ensuring their products meet the stringent safety and performance standards set by the government.
Furthermore, the South Korean government’s commitment to patient safety and the promotion of high-quality medical devices has been a significant driver for the regulatory affairs market. The government, through various regulatory agencies such as the Ministry of Food and Drug Safety (MFDS), has implemented comprehensive regulations, harmonized standards, and enhanced post-market surveillance mechanisms to ensure the long-term safety and performance of medical devices.
The growing trend of medical device exports from South Korea to international markets has also contributed to the demand for regulatory affairs services. As South Korean medical device manufacturers seek to expand their global footprint, they require support in navigating the regulatory requirements of various target markets, ensuring their products meet the necessary standards and obtain the required approvals.
Additionally, the increasing focus on digital health technologies and the integration of emerging technologies, such as artificial intelligence and connected devices, into the medical device landscape have created new challenges and opportunities for the regulatory affairs market. Medical device companies require specialized expertise to address the evolving regulatory considerations surrounding these innovative solutions.
Market Restraints
One of the primary restraints in the South Korean medical device regulatory affairs market is the complexity and evolving nature of the regulatory landscape. The continuous updates, harmonization of standards, and the introduction of new regulations can create challenges for medical device companies, particularly smaller or less-experienced players, in keeping up with the changing requirements.
Another key restraint is the limited availability of skilled and experienced regulatory affairs professionals in the South Korean market. The specialized knowledge and expertise required to navigate the intricate regulatory framework, as well as the ability to anticipate and address emerging regulatory trends, can pose a significant challenge for medical device companies seeking to strengthen their regulatory affairs capabilities.
The integration of emerging technologies, such as digital health solutions and AI-powered medical devices, into the existing regulatory framework can also act as a restraint. The regulatory authorities in South Korea may need to develop new guidelines, standards, and approval processes to accommodate these innovative technologies, which can create uncertainties and delays for medical device companies seeking to introduce such products.
Additionally, the COVID-19 pandemic has had a mixed impact on the South Korean medical device regulatory affairs market. While the increased demand for medical devices and the accelerated regulatory approval processes for pandemic-related products have created opportunities, the overall economic uncertainty and supply chain disruptions may have also led to temporary slowdowns in some areas of the medical device industry.
Furthermore, the potential for regulatory divergence between South Korea and other major markets, such as the United States and the European Union, can pose challenges for medical device companies seeking to navigate multiple regulatory frameworks and ensure global compliance.
Market Opportunity
The South Korean medical device regulatory affairs market presents significant opportunities for growth and expansion. As the country continues to focus on healthcare innovation, patient safety, and regulatory compliance, the demand for specialized regulatory affairs expertise is expected to remain robust.
One key opportunity lies in the support of South Korean medical device companies in their efforts to expand their global footprint. As these companies seek to export their products to international markets, the need for regulatory affairs services that can navigate the regulatory requirements of various target countries will continue to grow. Regulatory affairs service providers that can offer comprehensive cross-border compliance solutions will be well-positioned to capitalize on this opportunity.
Furthermore, the integration of emerging technologies, such as digital health solutions and AI-powered medical devices, into the medical device landscape presents a significant opportunity for the regulatory affairs market. As the regulatory authorities in South Korea develop new guidelines and approval processes to accommodate these innovative technologies, regulatory affairs service providers that can stay ahead of the curve and offer tailored solutions will be able to differentiate themselves and capture a larger share of the market.
Additionally, the growing emphasis on post-market surveillance and the ongoing compliance management of medical devices create opportunities for regulatory affairs service providers. As the South Korean government continues to strengthen its oversight and monitoring mechanisms, medical device companies will require support in ensuring the long-term safety and performance of their products, driving the demand for regulatory affairs services.
The South Korean government’s commitment to fostering innovation in the medical device industry, as evidenced by its various initiatives and funding programs, also presents opportunities for regulatory affairs service providers. By aligning their services and expertise with the government’s priorities, these providers can position themselves as strategic partners in the medical device ecosystem, contributing to the development and commercialization of innovative solutions.
Furthermore, the growing trend of medical device outsourcing and the need for regulatory affairs support among contract manufacturers and service providers can create additional opportunities for regulatory affairs service providers in the South Korean market.
Market Segment Analysis
Regulatory Strategy and Approval Segment The regulatory strategy and approval segment is a critical component of the South Korean medical device regulatory affairs market. This segment encompasses services that support medical device companies in navigating the complex approval processes, ensuring their products meet the necessary regulatory requirements, and obtaining the required certifications and registrations to access the domestic and international markets.
South Korean regulatory affairs service providers in this segment offer a range of services, including regulatory gap analyses, strategic planning, dossier preparation, and submission support for product registrations. They also provide guidance on regulatory changes, harmonization of standards, and the interpretation of evolving guidelines to help medical device companies stay compliant and streamline their market entry processes.
The demand for regulatory strategy and approval services is particularly high among medical device companies seeking to introduce innovative products or expand their presence in the South Korean market. These companies require specialized expertise to navigate the country’s regulatory landscape, address complex technical and clinical requirements, and ensure their products are approved in a timely manner.
As the South Korean government continues to emphasize the importance of patient safety and the quality of medical devices, the regulatory strategy and approval segment is expected to remain a key focus area for the medical device regulatory affairs market. Service providers that can offer comprehensive, efficient, and tailored solutions will be well-positioned to capture a larger share of this dynamic market segment.
Post-Market Surveillance and Compliance Segment The post-market surveillance and compliance segment is another crucial area of the South Korean medical device regulatory affairs market. This segment encompasses services that support medical device companies in monitoring the long-term safety and performance of their products, as well as ensuring ongoing compliance with evolving regulations and standards.
South Korean regulatory affairs service providers in this segment offer a range of services, including adverse event reporting, product vigilance, compliance audits, and regulatory gap assessments. They also provide guidance on post-market surveillance requirements, labeling and packaging updates, and the implementation of corrective and preventive actions to address any identified safety or performance issues.
The growing emphasis on post-market surveillance and the continuous monitoring of medical devices in South Korea has been a key driver for the demand in this market segment. The South Korean government, through its regulatory agencies, has implemented stringent post-market surveillance requirements to ensure the long-term safety and efficacy of medical devices available within the domestic market.
Medical device companies operating in South Korea are increasingly recognizing the importance of proactive compliance management and the need for specialized expertise in this area. Regulatory affairs service providers that can offer comprehensive post-market surveillance and compliance solutions, leveraging data analytics and digital technologies, will be able to differentiate themselves and capture a larger share of this dynamic market segment.
Regional Analysis
The South Korean medical device regulatory affairs market is primarily concentrated in the country’s major metropolitan areas, such as Seoul, Gyeonggi-do, and Busan. These regions serve as hubs for the medical device industry, housing a significant number of medical device manufacturers, importers, and distributors, as well as the country’s key regulatory agencies and research institutions.
The Seoul metropolitan area, in particular, has emerged as the epicenter of the South Korean medical device regulatory affairs market. The region is home to the Ministry of Food and Drug Safety (MFDS), the primary regulatory authority responsible for overseeing the medical device industry, as well as numerous industry associations, research centers, and leading medical device companies.
Beyond the capital region, other major industrial and healthcare centers, such as Gyeonggi-do and Busan, have also witnessed a growing concentration of medical device regulatory affairs service providers. These regions play crucial roles in the country’s medical device manufacturing and distribution, further driving the demand for specialized regulatory affairs expertise.
The regional analysis also highlights the importance of South Korea’s well-developed infrastructure, including extensive transportation networks and efficient logistics systems, in enabling the seamless integration and coordination of regulatory affairs services across different geographic areas. The country’s advanced telecommunications infrastructure, including the widespread availability of high-speed internet and 5G networks, also contributes to the effective remote collaboration and data exchange required in the regulatory affairs sector.
Furthermore, the regional concentration of research institutions, universities, and technology hubs in South Korea’s major metropolitan areas has fostered a vibrant ecosystem for the development and innovation of medical device technologies and regulatory frameworks. This ecosystem, combined with the presence of established medical device manufacturers and regulatory authorities, has created a favorable environment for the growth and expansion of the medical device regulatory affairs market within the country.
Competitive Analysis
The South Korean medical device regulatory affairs market is characterized by a mix of domestic and international service providers, each vying to capture a larger share of this rapidly growing market.
Domestic regulatory affairs service providers, such as KMC (Korea Medical Devices), CCQS, and Regulatory Affairs Consulting Korea (RACK), have established a strong presence in the market, leveraging their deep understanding of the local regulatory landscape and their ability to cater to the specific needs of South Korean medical device companies. These providers offer a range of services, including regulatory strategy development, product registration, and post-market compliance management.
In addition to the local players, several global regulatory affairs service providers, including Emergo (a UL Company), Kinematic, and Veristat, have also established a foothold in the South Korean market. These international companies bring a wealth of experience, technological expertise, and a diverse portfolio of services to the table, allowing them to offer comprehensive regulatory affairs solutions to medical device companies operating in South Korea and beyond.
The competitive landscape is further shaped by the presence of specialized consulting firms, contract research organizations (CROs), and technology-enabled service providers that play a crucial role in supporting medical device companies throughout the regulatory lifecycle. These companies often focus on delivering innovative, technology-driven solutions to address the evolving regulatory challenges faced by their clients.
The intensity of competition in the South Korean medical device regulatory affairs market has driven continuous innovation, service diversification, and the expansion of geographic reach. Service providers, both domestic and international, are constantly seeking to enhance their capabilities, leverage emerging technologies, and establish strategic partnerships to stay ahead of the curve and meet the changing needs of the medical device industry.
This competitive dynamic has ultimately benefited South Korean medical device companies, as they have access to a diverse range of high-quality, specialized, and tailored regulatory affairs services to support their efforts in bringing innovative products to market and ensuring long-term compliance.
Key Industry Developments
- Ongoing updates and harmonization of medical device regulations in South Korea to keep pace with technological advancements and changing healthcare needs
- Strengthening of post-market surveillance requirements and compliance management mechanisms to ensure the long-term safety and performance of medical devices
- Increasing focus on the integration of emerging technologies, such as digital health solutions and AI-powered medical devices, into the regulatory framework
- Collaboration between regulatory authorities, industry associations, and medical device companies to address evolving regulatory challenges and promote innovation
- Expansion of South Korean medical device companies into international markets, driving the demand for comprehensive cross-border regulatory affairs support
- Investments in the development of specialized regulatory affairs expertise and the enhancement of technology-enabled solutions to improve efficiency and compliance
- Initiatives to attract and retain skilled regulatory affairs professionals within the South Korean medical device industry
- Efforts to align South Korean medical device regulations with global standards and facilitate the seamless movement of products across international borders
Future Outlook
The future outlook for the South Korean medical device regulatory affairs market is highly promising, as the country’s commitment to healthcare innovation, patient safety, and regulatory compliance continues to drive the growth and evolution of this dynamic sector.
As the South Korean medical device industry continues to develop cutting-edge solutions and seek global expansion opportunities, the demand for specialized regulatory affairs expertise will remain robust. Medical device companies will require support in navigating the complex and evolving regulatory landscape, ensuring their products meet the necessary standards, and obtaining the required approvals to access both domestic and international markets.
The integration of emerging technologies, such as digital health solutions and AI-powered medical devices, into the medical device landscape will present both challenges and opportunities for the regulatory affairs market. Regulatory authorities in South Korea will need to develop new guidelines and approval processes to accommodate these innovative technologies, and regulatory affairs service providers that can stay ahead of the curve and offer tailored solutions will be well-positioned to capitalize on this trend.
The growing emphasis on post-market surveillance and ongoing compliance management will also continue to shape the future of the South Korean medical device regulatory affairs market. Medical device companies will increasingly require support in monitoring the long-term safety and performance of their products, as well as ensuring their continued compliance with evolving regulations and standards.
Furthermore, the South Korean government’s commitment to fostering innovation in the medical device industry, through various initiatives and funding programs, will create opportunities for regulatory affairs service providers to align their expertise and services with the government’s priorities. By positioning themselves as strategic partners in the medical device ecosystem, these providers can contribute to the development and commercialization of innovative medical solutions.
The expansion of South Korean medical device companies into international markets will also drive the demand for comprehensive cross-border regulatory affairs support. Regulatory affairs service providers that can offer seamless solutions for navigating the regulatory requirements of various target markets will be able to capture a larger share of the growing global opportunities.
Overall, the future outlook for the South Korean medical device regulatory affairs market is highly positive, with the potential for sustained growth, technological innovation, and the emergence of new, game-changing regulatory solutions that cater to the evolving needs of the medical device industry both within the country and globally.
Market Segmentation
- Regulatory Strategy and Approval
- Product Registration and Certification
- Regulatory Gap Analysis and Compliance Assessments
- Dossier Preparation and Submission Support
- Post-Market Surveillance and Vigilance
- Adverse Event Reporting and Investigations
- Labeling and Packaging Compliance
- Regulatory Change Management
- Cross-Border Regulatory Affairs Solutions
- Digital Health and AI-Powered Medical Device Regulatory Support
- Regulatory Training and Capacity Building
- Regulatory Affairs Consulting and Advisory Services
- Regulatory Affairs Outsourcing and Contract Services